5 Things About the Votiva Vaginal Rejuvenation Treatment
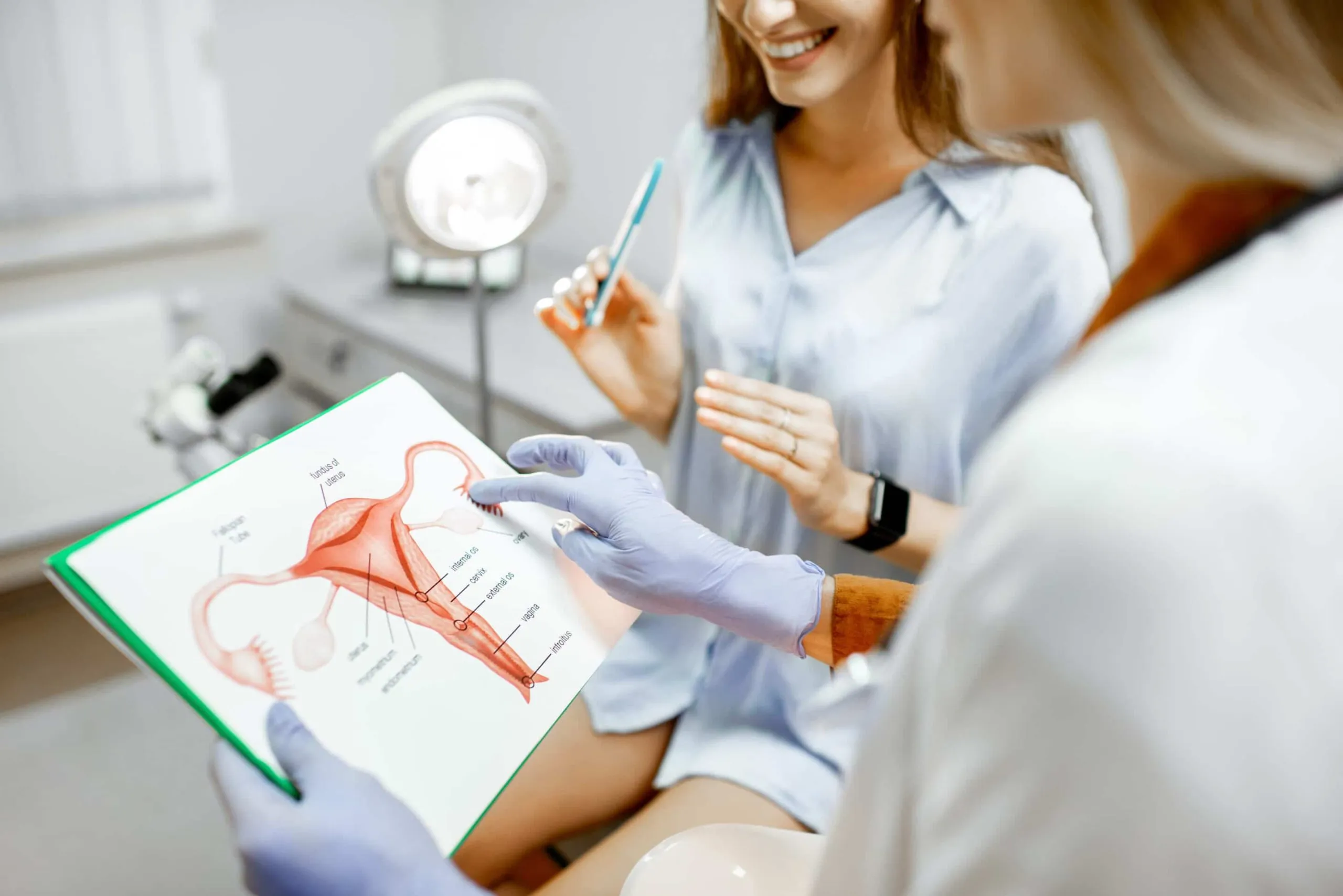
With age, menopause, and childbirth, the vagina undergoes a lot of changes. These changes include laxity or “stretched out” tissues, decreased lubrication, decreased sensitivity, painful intercourse, prolapse, and urinary incontinence. One form of treatment you should absolutely know about if you’re experiencing any of these symptoms is Votiva. Here are five facts you should know about this amazing treatment